Cell replacement therapy for parkinson’s disease

The idea behind cell replacement therapy(CRT) for PD is pretty simple: lack of mobility in PD is the result of the dysfunction and death of a specific kind of cell in the midbrain. While there are a few other things that go wrong in PD, the progressive loss of motor skills is the biggest problem most diagnosed face. Since we are reasonably sure that this lack of mobility results from the impairment and death of dopamine-producing cells in an area of the midbrain called the substantia nigra, why not try to replace those cells?
A group of iPS(induced pluripotent stem cells) cells grown from human skin tissue at Osaka University
Replacing those cells is one of three core problems that each person diagnosed with PD needs to address. They are:
1. Keeping remaining cells healthy
Once diagnosed, most people have already lost production of 50-80% of dopamine in their midbrain. The problem then is to stop further disease progression by figuring out how to get rid of everything that might be harming the remaining 20-50% of cells while giving their body everything it needs to keep those cells alive and active.
2. Clearing clogged cells
Of that 50-80 % of non-dopamine producing cells, a portion is still alive, they are just not doing their job, producing dopamine. This impairment is a result of a range of interrelated factors that harm the cells and eventually lead to their death. Most researchers believe the problem can be boiled down to the clumping of a misfolded protein called alpha-synuclein. Many different methods are being tried in labs around the world to clear these clumps and stop more from accumulating. But this might only be part of the story since a wide variety of other factors also lead to cell death.
3. Replacing dead cells
Then we come to what to do about all of those dead cells. A couple of different options are being considered to get the brain to stimulate the production of new neurons or replace the function of dead ones. However, the most promising therapy being developed is stem cell therapy, now commonly referred to as cell replacement therapy. It works by placing new dopamine producing neurons into the part of the brain where the dead neurons used to release dopamine.
If a patient manages to address problems one and two they might have no need for CRT. The reason for this is that he or she can likely rescue a considerable portion of the damaged but still living cells and thereby bring dopamine production back to a level that allows for normal movement. CRT will generally be for people who have had PD for a longer time and whose remaining healthy cells plus the rescued ones together are not capable of providing enough dopamine.
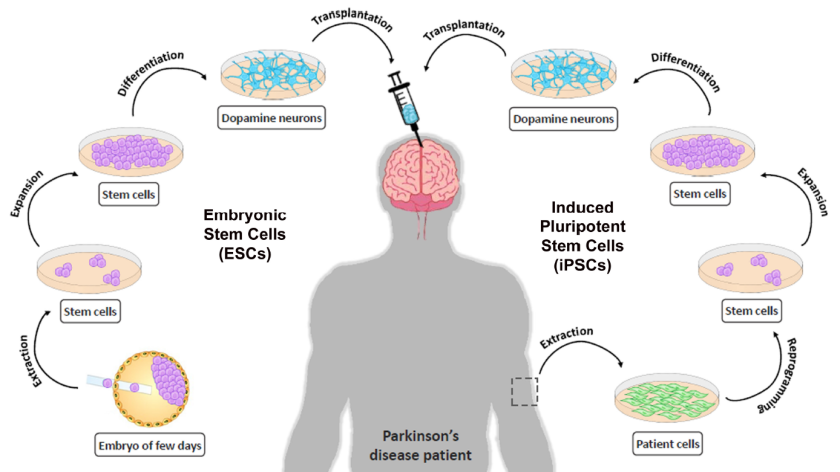
The late 80’s and 90’s saw a number of CRT trials for Parkinson’s disease with mixed results. But we now have a much better understanding of what kind of cells to use, how to culture and store those cells, how to implant them, and who this therapy would be best for.
We also now have iPS cells (induced pluripotent stem cells). Discovered in 2006, these are cells that have been chemically reprogrammed, usually from adult skin tissue, back into pluripotent stem cells. (Pluripotent means they are capable of becoming almost any cell in the body). Using these cells for transplantation has two major advantages. One, it eliminates the need for potentially harmful immuno-suppressors. Two, it has none of the ethical issues that come with using fetal stem cells. But iPS cells are much more expensive and technically difficult to produce.
Despite all the progress made, cell replacement therapy is still very controversial and fraught with all sorts of technical issues. Luckily, CRT for PD is one of the only fields of medical science where the top labs around the world are cooperating with each other. An international consortium of labs has come together under a name that sounds like it was ripped out of a Marvel comic, the GForce-PD. Each lab in the GForce-PD aims to bring CRT for PD to clinical trial within the next few years.














Blood Test Might Predict Pregnancy Due Date and Preterm Birth